“I think when people tell the public that there's going to be a vaccine by the end of 2020, they do a grave disservice to the public ... Let me just give you one data point. In the last quarter century, there have only been seven, truly new vaccines introduced globally at the clinical practice. Merck has four, the rest of the world has three.” - Ken Frazier, CEO of Merck
The level of optimism in equity markets and society in general regarding the progress on developing a vaccine for COVID-19 has certainly been increasing.
The world is desperate for vaccine updates and is putting the recovery of the economy and the reduction in the health crisis on the line. So much so, it’s like the vaccine is almost fully developed and trialled.
Personal experience with vaccines
I am not sure how many of this readership has been personally exposed to vaccines. Sure, the flu shot is common, but if people think the vaccine for COVID-19 will be like receiving a flu shot, they could be in for a very rude and thought-provoking wake-up call.
Not all vaccines are as simple and benign as the influenza virus.
Growing up in Papua New Guinea, my entire family was given the smallpox vaccination. My father at the time in the early 1970s was 35 and he took us to the doctors to get jabbed. The negative side-effects of the smallpox injection were known at the time but we had little choice as it was a legal requirement to be inoculated prior to travelling to PNG.
The impact it had on my dad was horrible. He spent four days in hospital and three weeks in bed. He was very, very sick. If this type of side-effect is what could be expected from any COVID-19 vaccination, how many people will want a vaccination and can the hospitals cope with the negative side-effects?
The global search
The search for a vaccine is front of mind in the pharmaceutical industry, with some 160 individual programmes under way. In recent times, Moderna Inc has been in the news with some positive results from a Phase 1 clinical trial across 45 healthy adults. All participants in the trial showed an antibody response.
Antibodies are the proteins the body makes to fight infection. Being safe to use in 45 people is not the same as being safe for say 20 or 200 million people that would need vaccinations in the US alone, but the market is rewarding Moderna.
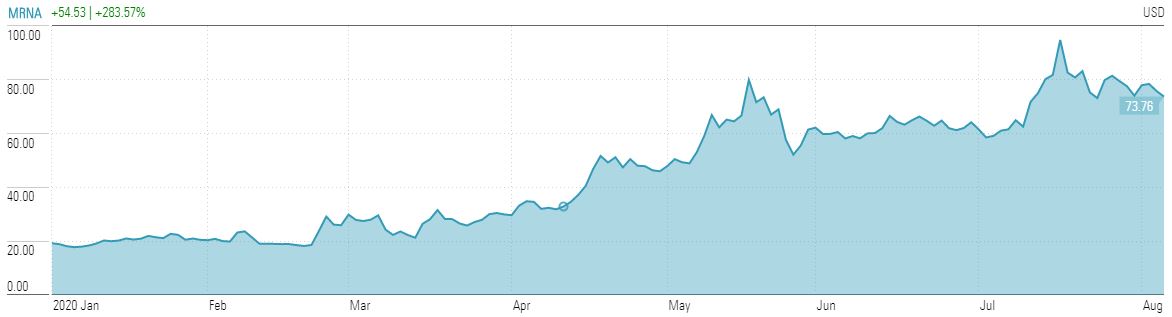
Source: Morningstar Direct
A reality check on development time
Ken Frazier, CEO of pharmaceutical giant Merck, was interviewed recently by Professor Tsedal Neeley from Harvard Business School. If there was ever a rational discussion on the topic in my view, it was this.
I will summarise his interview with the following bullet points.
- Developing a vaccine takes time, a lot of time. The fastest vaccine ever brought to market was for the epidemic parotitis (‘mumps’). It took Merck four years to produce this vaccine.
- The most recent vaccine created for a large viral outbreak was for the Ebola virus, which took 5.5 years.
- In the past 25 years there have been only seven truly new vaccines introduced globally. By new, that means that they were effective against a pathogen for which there had previously been no vaccine. Merck has developed four of those seven and the rest of the world three. There has been an enormous amount of work done in the field of prevention. Despite all this work, the world has been trying to develop a vaccine for AIDS since the early 1980s, and so far, without success.
- Developing a vaccine requires vigorous scientific assessment. Vaccines must be safe, effective, and durable. No one knows if any of the 160 programmes will produce a vaccine that is effective. This vaccine must work on billions of people.
- Lots of vaccines in the past have stimulated the immune system (just like the Moderna trial vaccine) but ultimately did not confer protection.
- When politicians suggest there will be a vaccine available by the end of 2020, they are doing the public a “grave disservice”.
- We do not want to rush the vaccine before rigorous science is done. We do not have a good history of introducing a vaccine in the middle of a pandemic. The swine flu vaccine did more harm than good.
- While we are working hard on a vaccine, the best preventative measures to limit the spread and infection of COVID-19 are good hygiene, wearing a mask and social distancing.
- The bigger challenge to developing a vaccine is distributing it to where it is needed most. In a time of ultra-nationalism, countries want to take whatever is available and use it in their own population first rather than offering it to populations globally at greatest risk.
- Developing a vaccine for 7 billion people has never been done before. Delivering it to 7 billion people is an enormous logistical challenge, especially to those communities who cannot afford it.
- This is a global pandemic. Unless all of us are safe then none of us is safe.
- The mobility of the world’s society poses a real problem. The EU has barred Americans travelling to Europe for a reason. Americans are not doing the things required to suppress the epidemic. Americans value liberty. It has been a strong theme through US politics for 200 years, largely because the US has two big oceans protecting it. This virus does not care about liberties. If people exercise liberty at the personal expense of others, then we cannot control this pandemic.
- America is 4% of the world’s population and 25% of the world’s infections. That’s scary.
- We need politicians with enough integrity to tell the truth. This time next year we will still be experiencing what we are experiencing now. Be prepared for that.
With these sage words lingering in your mind, let’s hope that when a Phase 3 trial for the Moderna vaccine gets under way, a trial that will involve thousands of patients, the results of the safety and efficacy will be such that a reliable vaccine can be developed.
Just be conscious that it will be a miracle if it is developed before the end of 2020, and if you believe Ken Frazier, 2025 is a more realistic time frame.
(The full video interview and transcript are here).
Rod Skellet is an Equities Investment Strategist at Mason Stevens. The views expressed in this article are those of the author as at the date published and are subject to change based on markets and other conditions. This article contains general information only and does not consider your individual circumstances.